Research conducted by the Economist Intelligence Unit suggests that the sector will see growth running at an annual rate of more than 5% between now and 2018 on the back of a strengthening global economy.
An ageing population is expected to generate increasing demand for life science products to treat a range of age related illnesses, such as Alzheimer's, diabetes and arthritis, while new portable medical technology will bring more effective health monitoring to patients living in some of the remotest regions of the world.
Meanwhile, the market for medical technology is expected to grow by 5% per year between now and 2020, with in vitro diagnostics, cardiac devices and diagnostic imaging technologies the three largest segments. But the fastest growing sector is forecast to be neurology devices.
There are, however, two opposing pressures impacting the development of medical devices, according to Arun Veeramani, National Instruments' senior marketing manager for embedded systems. "The first is the need to produce safe, high quality devices that are suitable for patient care. The second is the growing pressure on companies to reduce development time so they can establish an early position in what is a very competitive market."
The cost of developing new devices can be prohibitive and the risks associated huge.
"That pressure to innovate more quickly reflects the pressures traditionally associated with the consumer sector. That mentality is now forcing companies to not only produce working prototypes more quickly, but also to decide whether a project is a go or no-go at an earlier stage."
The pressure on the domain expert in life sciences and on the electrical engineer is certainly immense.
"In general, for those developing medical devices, it's vital that they see increased throughput, via greater automation, and a reduction in costs. They also need to have confidence in the test environment, especially if the product under development is portable. Reliability in the field is paramount if the device is being deployed in remote areas."
A crucial new dynamic in the design cycle has been, according to Veeramani, the breaking down of what he describes as the 'wall' between design and test.
"We've seen this wall crumble as both sets of engineers access the same code and better understand the work of the other," he says.
"NI has played its part in breaking down that wall. LabVIEW, for example, is a single platform that can support design, prototyping and test. A highly reconfigurable I/O (RIO) architecture, combining Xilinx FPGAs and PC based technologies in a platform, it is suited for onboard processing and real-time analysis."
Among projects undertaken by NI was one with Santec to increase the imaging speed and reduce the size of an optical coherence tomography (OCT) imaging system.
"OCT is a non invasive imaging technique that enables the visualisation of tissue with resolution similar to that of some microscopes," explains Veeramani.
There has been increasing interest in the use of OCT because it provides greater resolution than techniques such as magnetic resonance imaging (MRI) or positron emission tomography (PET). The process uses a low power light source and the corresponding light reflections to create images – a method similar to ultrasound, but which uses light not sound.
In Swept Source-OCT (SS-OCT) applications, a laser scans a sample, a fast A/D converter acquires the data and produces a tomographic image.
The system needs to be capable of high speed acquisition, complex image processing and accurate control of the laser scanning. The acquisition and control portions of the system have to be tightly synchronised if they are to achieve a satisfactory level of performance.
 |
Obtaining the final image requires significant processing power, including fast Fourier transforms, interpolation and DC offset calculations.
To prototype the new architecture, Santec used NI's FlexRIO modular hardware programmed using the LabVIEW FPGA Module.
"This is a graphical design language that allows the FPGA circuitry to be designed without needing to know VHDL coding. "It combines interchangeable, customisable I/O adaptor modules with a user programmable FPGA module in a PXI or PXI Express form factor."
For the I/O, Santec used a custom adapter module combining a 100Msample/s 12bit A/D converter for acquisition with 50ksample/s 12bit D/A converter for the laser scanner control.
"Prototyping the system meant a working solution was created quickly and it was possible to determine any changes that were needed," Veeramani explains.
"We saw a significant speed-up when we moved processing to the FPGA from the PC, which enabled significant improvement in video display rates – from 10frame/s per second to 40frame/s."
In the conventional system, two devices were needed. By contrast, the new platform combined the acquisition and control I/O in one module, using the FPGA to synchronise both functions, making the device easier to build, cable and configure.
Because of a reduction in size the device could be made portable, opening it up to use in a range of applications.
Another project was with the Healthcare Technology Innovation Centre (HTIC) of IIT Madras; since its formation in 2011, a leading source of medical research and development in India.
With a focus on developing affordable healthcare technologies to meet clinical needs, HTIC worked with NI to develop an affordable and non invasive method of measuring arterial stiffness – cardiovascular disease is a significant killer globally and affects 45million Indians.
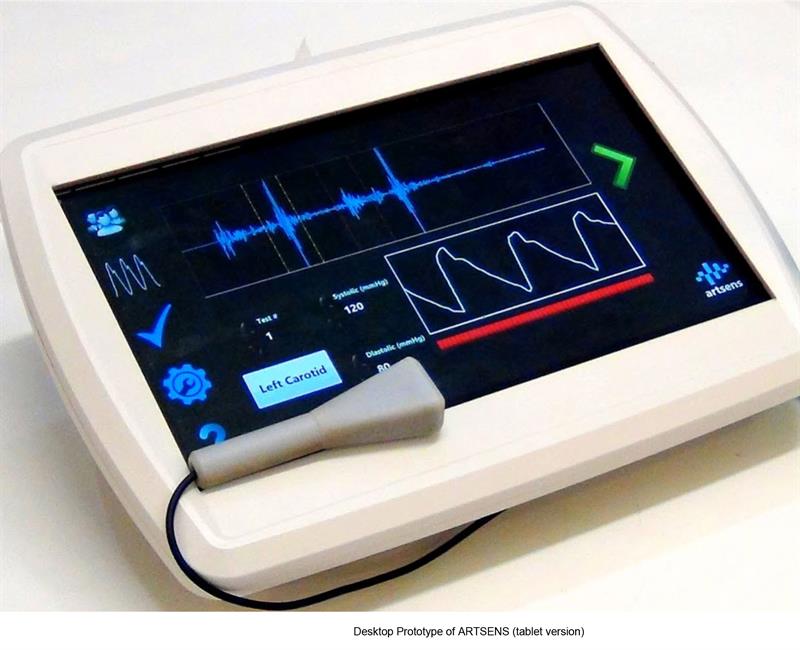
HTIC developed ARTSENS – arterial stiffness evaluation for non-invasive screening – an image free technology that investigates arterial wall dynamics and performs non invasive measurements of arterial stiffness using a high frequency transducer to capture the dynamics of the arterial wall.
 |
"The arterial wall's motions can be tracked and the user can obtain a distension waveform, measure the end diastolic diameter and, along with the measurement of blood pressure, can calculate arterial stiffness," explains Veeramani.
HTIC developed miniaturised hardware modules for transducer excitation and synchronised data acquisition in house and integrated them with intelligent computing modules for real time processing and online signal analysis to develop a prototype. HTIC is currently developing a handheld version of ARTSENS.
"The use of NI hardware and LabVIEW software allowed much quicker experimental setup and allowed the research team to capture the echo signals and focus on understanding the signal characteristics, without getting bogged down with the setting up of the experiment," said Veeramani.
While testing to meet performance requirements can be time consuming it needs to be carried out efficiently and effectively. As the divide between the designer and tester blurs, new products can be brought to market more quickly, on budget and, crucially, without compromising on safety.













