There are bio-sensors that measure real-time heart rate, blood pressure, body temperature, skin moisture, blood oxygen levels, respiration rate, and ECG (electrocardiogram). All help detect any irregularities before they develop into dangerous health problems.
Wearables are also used with patients returning home from hospital to monitor their post-operative recovery and watch for any complications. This helps ease the health provider strain by letting the patients return home sooner, but still monitor their conditions closely.
For those with diabetes, glucose sensors under the skin check blood sugar levels and connect to smartphones for easier readings. There are also fitness trackers to measure exercise levels and calorie intake and to analyse sleep patterns to encourage healthier lifestyles. Wearable devices are also used to monitor loved ones and summon assistance in the event of a fall or medical emergency.
These small devices only continue to grow in popularity and function and have a big impact on patient health and safety.
The challenge of small
As these wearable devices get more complex and powerful, there is a challenge to keep them small, discrete and comfortable for patient use. This also means the internal PCBs (printed circuit boards) that power the devices must shrink too.
The result is devices filled with densely-packed and multi-layered PCBs that are sometimes difficult to clean during production. Contaminated or dirty PCBs are vulnerable to a number of problems including parasitic leakage, electrochemical migration, delamination, dendrite growth and shorting.
Reductions in pitch between conductors along with the increased use of leadless, zero-clearance or bottom-terminated components increase the probability that contamination, like active fluxes or flux residue, gets trapped on the PCBs. Add in other contaminants, like ink and fingerprints, and the threat of unreliable PCB performance and possible catastrophic field failure escalates.
It is vital to clean PCBs to ensure they function as intended. It is of equal importance to plan the cleaning fluid and cleaning method before PCB production starts.
Planning ahead for a cleaning process while other decisions about coatings and solder pastes are made helps ensure the chosen cleaning fluid and technique are successful. It also prevents PCB fabricators from scrambling at the end of the production line to find a cleaning solution that might work on a particularly difficult-to-remove contaminant or flux residue.
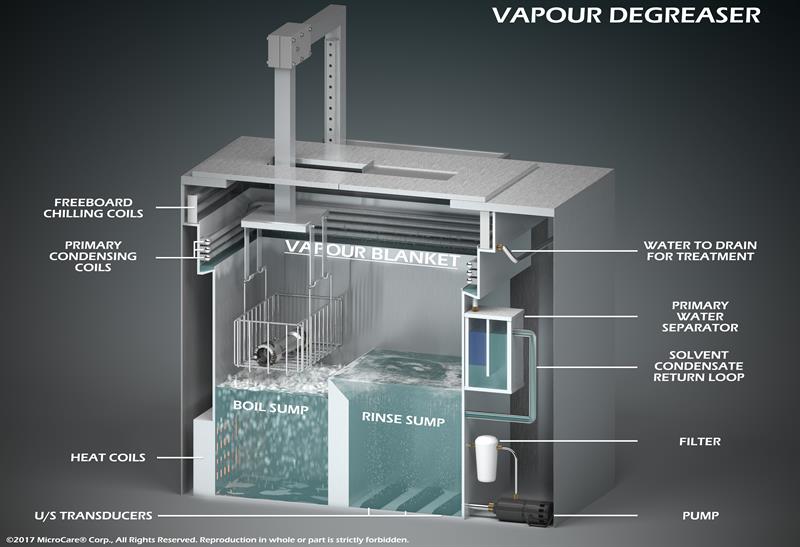
Above: Vapour degreasers clean, rinse and dry electronics in one step
Choosing a cleaning fluid
PCBs can be exposed to a variety of different contaminants during the production process. They are categorised into four main groups: insoluble particulate, organics, inorganics and water.
Insoluble particulate is a polar contaminant that cannot be dissolved by water or a cleaning fluid. Common particulate contamination includes things like dust, cloth fibres and metal chips. Particulate electrostatically bonds to PCB surfaces and requires an electrostatic polar cleaning fluid. A dense cleaning fluid that contains slightly conductive molecules will break the static bond of the contaminant and displace, or float, the particulate off the PCB substrate. Sometimes agitation helps enhance the cleaning fluid’s effectiveness as well.
Organic or non-polar contaminants include rosin solder pastes and fluxes that come in grades of activation including R (Rosin), RA (Rosin Activated), RMA (Rosin Mildly Activated) and SA (Synthetic). Organic contaminants can also include non-polar oils and greases. Organic contamination can be dissolved and removed with mild to medium strength organic flux removers.
Inorganic, polar contaminants are the residue left by lead-free and no-clean fluxes and solder pastes. Inorganic contamination sometimes appears on PCBs as white residue which can be extremely difficult to remove. Inorganic contaminants usually require more aggressive flux removers to dissolve contaminants, especially the stubborn white residue. It is important in these instances that the material compatibility of the cleaning fluid is known before use. If a cleaning fluid is too strong, it may damage sensitive PCB materials such as soft plastics.
Water comes in contact with PCBs through the use of aqueous cleaning systems and is easily trapped in more densely populated circuit boards. Water is typically removed by air knives or other dryers. However, this may still leave moisture and allow water spots to form. Water contamination can be chemically removed using batch drying inside a vapour degreaser outfitted with a water separator.

Above: As wearable devices get more complex, the challenge is to keep them small, discrete and comfortable for patients
Choose a cleaning method
There are a number of ways to clean PCBs, from benchtop manual cleaning to in-line aqueous operations. However, as PCBs become more challenging to clean, PCB fabricators are turning to vapour degreasing as they realise the benefits of this tested and proven cleaning method.
Vapour degreasing is a simple process that is effective at removing contaminants. It also satisfies the economic, validation and regulatory requirements needed within the medical device manufacturing industry.
Vapour degreasers use a closed-loop system containing two chambers, the boil sump and the rinse sump. The boil sump contains a specially-formulated low-boiling, non-flammable cleaning fluid. The PCBs are immersed and cleaned inside the heated fluid. Once cleaned, the PCBs mechanically transfer to the rinse sump for final rinse and dry in more pure, uncontaminated fluid, or inside the fluid vapours themselves. The PCBs come out clean, dry, and spot-free. The process is simple, repeatable and easy to validate.
Many modern PCB cleaning fluids used inside the vapour degreaser have exceptional materials compatibility, making them suitable for cleaning delicate plastic parts or mixed-material printed circuit boards. They also have low surface tensions and high liquid densities. This allows the cleaning fluid to easily flow around tight-fitting and low-mounted components to clean under them thoroughly. It also enables the fluid to flow back out from under the components, preventing the fluid and the contaminants from getting trapped. This ensures all contaminant gets completely removed.
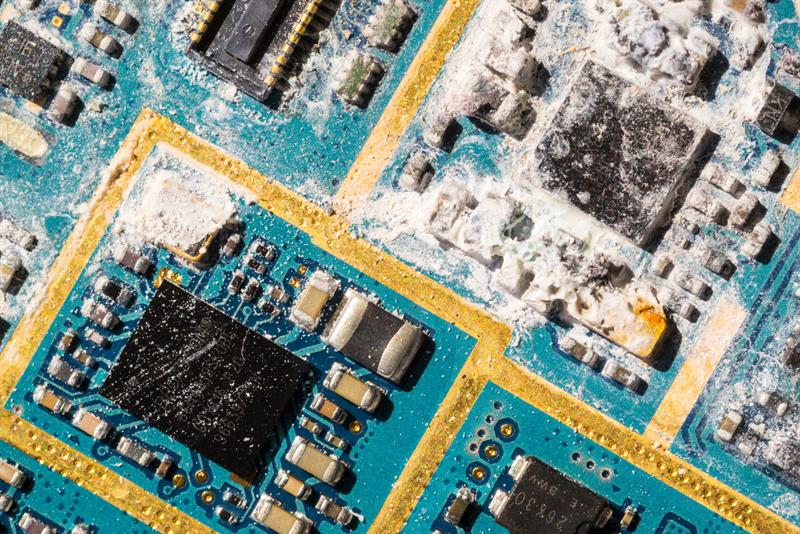
Above: Inorganic contamination sometimes appears on PCBs as white residue which can be challenging to remove
Importantly, vapour degreasing fluids are hostile to bacteria. So, by using this cleaning process it ensures a sterile cleaning environment. Vapour degreasing simplifies process control requirements for eliminating bioburden and offers an easy way to validate the PCB manufacturing process.
Find a cleaning partner
Wearable medical devices continue to gain in popularity as people become more proactive in maintaining their health. This is even more apparent since the COVID-19 outbreak, where more of our medical care is now remotely monitored and socially distanced.
As medical monitoring devices get smaller and more complex, cleaning their internal PCBs becomes more critical to ensure they perform without fail.
By planning PCB cleaning in advance, knowing the contaminant and choosing a trouble-free cleaning method, PCB fabricators can be well-prepared to produce clean, reliable boards.
Before deciding on a cleaning fluid or method, consult with a critical cleaning expert. They can suggest compatible and effective fluids for PCB cleaning success.
Author details: Emily Peck is a Senior Chemist at MicroCare













