In a bid to overcome this and prevent this initial loss of Li ions, scientists from the Korea Institute of Science and Technology (KIST) have developed an electrode pre-treatment solution that is capable of minimising this initial Li ion loss in graphite-silicon oxide (SiOx, 0.5 ≤ x ≤ 1.5) composite anodes.
After being dipped in the solution, the anode, which was composed of 50% SiOx, demonstrated negligible Li loss, enabling a full cell to exhibit near-ideal energy density.
Most commercial Li batteries utilise a graphite anode, but SiOx has been garnering significant attention due to its high capacity, which is 5-10 times greater than graphite. SiOx, however, also irreversibly consumes three times as much active Li as graphite. As a result, a composite electrode, consisting of a mixture of graphite and SiOx, has been promoted as an alternative for practical next-generation anodes.
While there was a corresponding increase in the capacity of graphite-SiOx composite electrodes at higher ratios of SiOx, there was also an increase in loss of initial Li. As a result, the ratio of SiOx content in a graphite-SiOx composite electrode had to be limited to 15%, as increasing the ratio to 50% would result in an initial Li loss of 40%.
To counter this the research team at KIST has developed a process where the electrode is dipped into a unique solution to mitigate Li consumption by the SiOx electrode. The team then applied this process to a graphite-SiOx composite material with significant commercialisation potential.
The team found that the pre-treatment solutions developed previously would cause the unintended insertion of solvent molecules with Li ions into the graphite, owing to the graphite’s versatile intercalation capability. This intercalation of large solvent molecules resulted in the structural breakdown of the graphite-SiOx composite electrode. To prevent electrode failure, the researchers developed an alternative solution using a weakly solvating solvent to reduce the interaction between the solvent and the Li ions. This solution enabled the selective insertion of Li ions into the active materials, ensuring a stable supply of additional Li to the graphite-SiOx composite electrode.
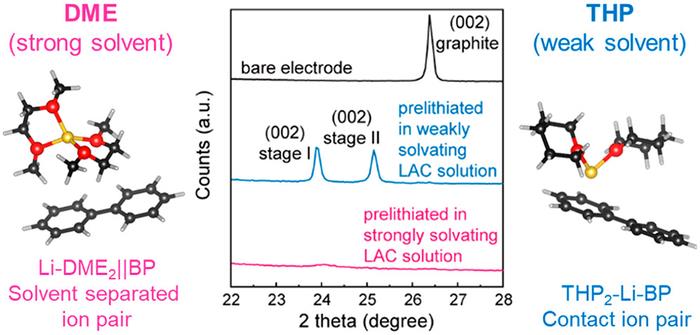
The initial Li consumption was completely prevented after the graphite-SiOx electrode was immersed in the solution developed by the research team for approximately 1 minute, even at a 50% SiOx ratio. Consequently, the electrode showed a high initial efficiency of nearly 100%, indicating negligible Li loss (≤ 1%) in the initial charge. Electrodes developed through this process had a capacity 2.6 times higher than conventional graphite anodes, while also maintaining 87.3% of the initial capacity after 250 charge-/discharge cycles.
According to Dr. Minah Lee from KIST, “As a result of this study, we should be able to increase the SiOx content in graphite-SiOx composite anodes to over 50%, as opposed to the 15% ratio allowed by conventional materials, making it possible to produce lithium ion batteries with a greater capacity and improve the mileage of future EVs.”
Dr. Jihyun Hong, a co-researcher at KIST, went on to add, “The technology is safe and suitable for mass production, and therefore is likely to be commercialised.”













