Microvisk secures £6million to launch diagnostic SmartStrip
1 min read
Microvisk Technologies has secured £6million in funding to develop the 'world's first' diagnostic SmartStrip, a MEMS based handheld system which monitors the blood clotting status of patients taking the drug Warfarin. This is the third successful funding round for the company, which has secured £10.5m in the past 12 months, and thought to be the largest amount ever raised by a UK life science company.
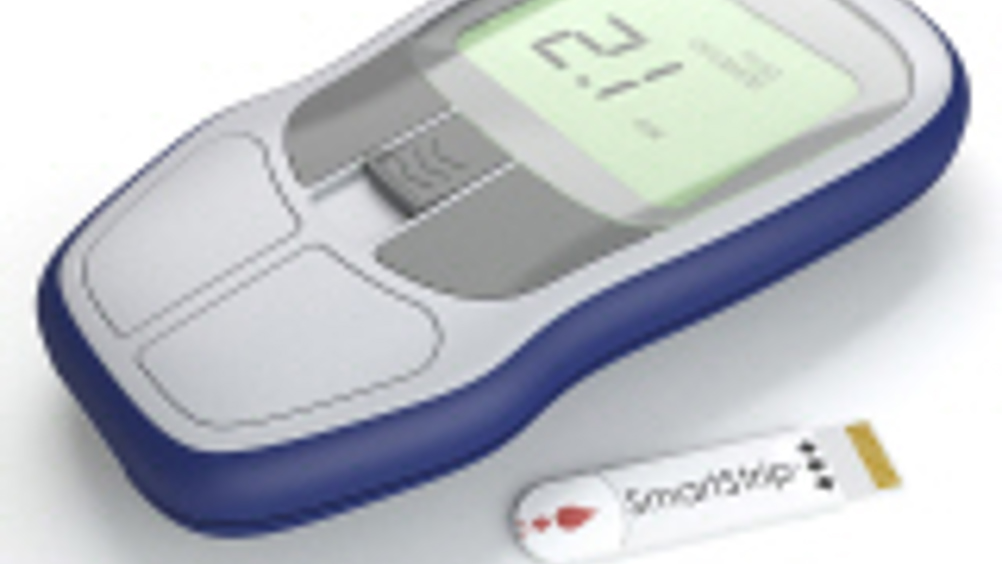
John Curtis, chief executive officer of Microvisk, said: "We are delighted to have raised the funding needed to gear up for the manufacture and launch of our SmartStrip product in the UK this autumn. This funding round represents a real milestone for us as we also start to prepare for expansion into the German and US markets over the following year."
Although originally created as a movement system for nanorobots, the SmartStrip enables patients to test their blood clotting capacity at home, in the same way as diabetics test for glucose. The disposable device uses embedded sensors to work out the clotting speed of blood from a finger prick sample. The results are then displayed on a handheld reader. The clotting speed of the patient is measured by tiny multilayered paddles on the surface of the strip and a memory chip ensures the device is calibrated to provide the highest levels of accuracy.












