The alloy of two parts copper, one part manganese and one part tin developed a field stronger than that of nickel, although changes in temperature could weaken the field dramatically.
Until the advent of quantum mechanics, the reasons why alloys in specific ratios acquired magnetic properties eluded materials scientists and physicists. They did find that critical elements came from three neighbouring regions towards the middle and right of the periodic table.
Quantum mechanics revealed the contribution made by symmetry to the properties of materials. In his seminal lecture series on foundational physics, Dr Richard Feynman made the point that, in classical theory, ideas such as conservation of momentum have to be treated as fundamental principles. Symmetry in quantum physics provides the basis for these apparent building blocks of nature because asymmetry shows when states have genuinely changed.
Heusler’s alloys with baffling properties were the start of the discovery of several classes of material where symmetry is fundamental to controlling the behaviour of the electrons that move through the crystal lattice. The key to these novel properties lies in the interaction between the symmetry of ions held in a well-defined crystal lattice and the often complex energy-band structure of the electrons they share.
The simple molecule benzene – a ring of six carbon atoms with one hydrogen atom attached to each – puzzled scientists for centuries. According to conventional chemistry, the ring should consist of alternating single and double bonds between the carbon atoms. Double bonds are normally shorter than single ones so, viewed through the lens of valence theory, benzene with a ring of alternating single and double bonds should not be perfectly hexagonal. But it is.
In its highly symmetric form, the electron orbitals around the carbon atoms in the ring degenerate, electrons become practically free of mass and can move at relativistic speed around the ring. Paul Dirac and Hermann Weyl derived relativistic versions of the Schrödinger equation to predict what would happen under these conditions. In a chemical like benzene, the equations predict cone-shaped orbitals emerging from the top and bottom of each carbon atom. In benzene, they merge and electrons move freely through these molecule-wide orbitals (see fig 1).
If you join a lot of benzene molecules together, displacing the carbon-hydrogen bonds with carbon-carbon, you wind up with graphene. In graphene, the cones link to create a pair of orbitals that give the 2D molecule a resistivity lower than that of silver at room temperature and a number of other exotic properties that could be harnessed in electronics.
The ‘Dirac cones’ identified in graphene are being found in other materials, but not in obvious places. Silicon and germanium – from the same group in the periodic table as carbon – do not flatten into ribbons well enough to be good candidates. Instead, the cones turn up in 2D molecular materials such as molybdenum sulphide (MoS2), which some researchers believe has the ability to become a successor to silicon MOS technology – and a convenient acronym. But the properties of other compounds that exhibit Dirac cones may prove to be more useful.
Heusler’s discovery pointed to another rich source of materials with Dirac cones based on ionic crystals. Some Heusler alloys are topological insulators. While they have surfaces with connected Dirac cones, their crystal core is an insulator – although they are not always very good insulators. Their Heusler heritage marks them out from graphene-like materials in the way they interact with the spin of electrons.
The surface does not just conduct electrons with the ease of graphene; it also controls movement based on their spin. In an idealised case, spin-up electrons will move in one direction; spin-down electrons will move the other way. This makes them candidates for generating and controlling spin-polarised currents, providing a simpler method for building spintronic circuits than is possible using more conventional materials.
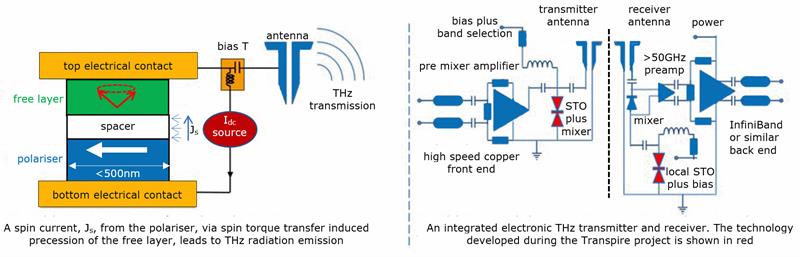 Figure: Trinity College Dublin, as part of the Transpire project, is exploring how 'half metals' could be used to generate and detect THz radiation
Figure: Trinity College Dublin, as part of the Transpire project, is exploring how 'half metals' could be used to generate and detect THz radiation
Heusler alloys point to another type of material with odd magnetic properties – the half-metal. Though easily confused with ’semi metals’ –which describes bismuth and antimony – the half-metal has a distinctly split personality. For one spin orientation, it is an insulator; for the opposite spin, electrons move as they would in a metal. Another odd property is that the magnetic moment is quantised. In principle, the moment can be zero, rendering the compound seemingly impervious to magnetic fields. The tricky part is making a zero-moment half-metal.
Half metals that are close to Heusler alloys are relatively simple to predict. An alloy of manganese, scandium and aluminium is one candidate. There is, however, one problem: materials with right ratio of elements stubbornly refuse to form the body-centred crystal structure needed to yield a working half-metal.
An alternative, which widens the search space for half-metals, is to exploit the strain engineering techniques used in CMOS processes to force thin films of these materials into the right alignment. One candidate is a combination of manganese, ruthenium and gallium dubbed ‘Mister G’ by Professor Michael Coey of Trinity College, Dublin. His group has built tunnel junctions of the kind used in magnetic memories – one of the possible applications for half-metals.
The big advantage of a half-metal in a tunnel junction is that it only allows electrons with the right spin to flow through. A single layer of Mister G could replace three or four carefully deposited layers in a tunnel junction. Even so, Prof Coey argues, the main application may not be in memories,but in THz oscillators and detectors, potentially overcoming the current speed limit afflicting chip-to-chip communication. The EU-funded Transpire project, which sprang from an initial collaboration between Trinity College and the Materials Research Institute at the Helmholtz-Zentrum Dresden-Rossendorf, is working on harnessing rapid precession inside materials with novel spin properties to generate and detect THz transmissions (see fig 2).
There is a problem with putting Mister G and his relatives into action. Manganese diffuses readily into adjacent layers, disrupting the carefully grown crystal. A thin layer of an unreactive material, such as tantalum, seems to fix the problem at a little extra cost. But theoretical work on topological insulators may lead to a way to avoid manganese entirely.
An international team from Princeton University, University of the Basque Country and the Max Planck Institute has developed a modelling technique that could discover useful topological insulators among other symmetry-influenced materials. But, even with an expanded palette of candidate materials, manufacturing-related problems remain.
No crystal is perfect and defects such as missing or substituted atoms break down the distinction between surface conductor and internal insulator. Using a 30Tesla magnet, PhD candidate Eric de Vries at the University of Groningen found it was easy to mistake current flowing through these defects for those that are meant to be only flowing at the surface. This compromises the ability of the material to separate electrons with opposite spins.
Techniques such as atomic layer deposition, already used to create the thin films needed for MRAM tunnel junctions, will help keep defects to a minimum. But there is still some way to go before topological insulators and their near relatives start to supplant the existing options for magnetic memories and spintronic circuits. As more candidate materials emerge and are probed, they may yield one that has the same impact that silicon and its oxide had on the first half century of microelectronics.












