The problem lies in their resistance at the electrode/solid electrolyte interface is too high, hindering fast charging and discharging.
The scientists fabricated all-solid-state batteries with extremely low interface resistance using Li(Ni0.5Mn1.5)O4(LNMO), by fabricating and measuring their batteries under ultrahigh vacuum conditions, ensuring that the electrolyte/electrode interfaces were free of impurities.
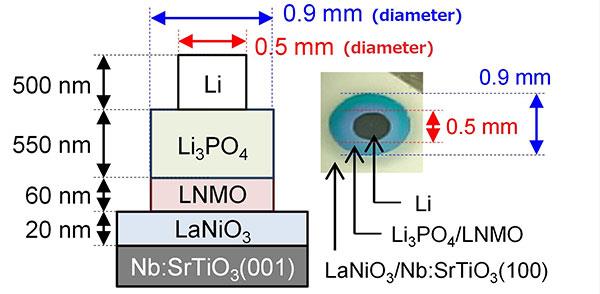
| Figure 1. Structure of the thin-film all-solid-state batteries. The batteries were made by stacking various layers via thin-film deposition methods. The LNMO/Li3PO4 interface showed spontaneous migration of Li ions and had an unprecedentedly low resistance. |
The structure of these all-solid-state batteries is shown in figure 1. After fabrication, the electrochemical properties of these batteries were characterised to shed light on Li-ion distribution around the interface. X-ray diffraction and Raman spectroscopy were used for analysing the crystal structure of the thin films comprising the batteries. Spontaneous migration of Li ions was found to occur from the Li3PO4 layer to the LNMO layer, converting half the LNMO to L2NMO at the Li3PO4/LNMO interface. The reverse migration occurs during the initial charging process to regenerate LNMO.
The resistance of this interface, verified using electrochemical impedance spectroscopy, was 7.6 Ω cm2, two orders of magnitude smaller than that of previous LMNO-based all-solid-state batteries and even smaller than that of liquid-electrolyte-based Li-ion batteries using LNMO. These batteries also displayed fast charging and discharging, the researchers add, managing to charge/discharge half the battery within just one second. Moreover, the cyclability of the battery showed no degradation in performance even after 100 charge/discharge cycles (see Figure 2).
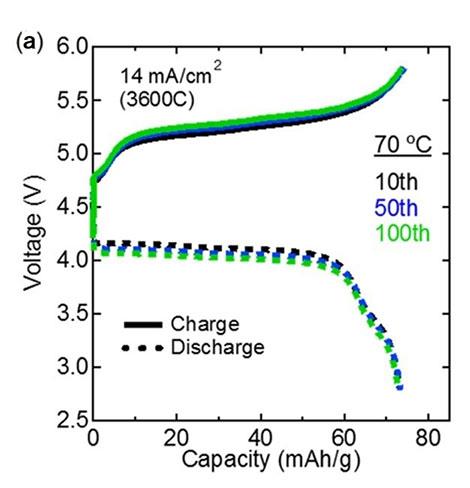
| Figure 2. Performance of the fabricated all-solid-state batteries. The (a) charge-discharge curves and the (b) cycling performance plot show that the performance of the fabricated all-solid-state batteries did not degrade after repeated use, demonstrating their excellent stability and the total reversibility of the reactions involved in charging/discharging. |
According to the team, Li(Ni0.5Mn1.5)O4is a promising material to increase the energy density of a battery, because the material provides a higher voltage. The scientists hope that these results will facilitate the development of high-performance all-solid-state batteries, which could revolutionise modern portable electronic devices and electric cars.













