In the cathode of metal-air batteries, oxygen is reduced to metal oxide or water. Platinum is a popular catalyst, but expensive. In the study, the researchers used perovskite as a substitute for platinum and reported that perovskite’s catalytic activity was ‘dramatically enhanced’ by adding a conducting polymer called polypyrrole.
"Because the oxide-polypyrrole complex is made by a simple operation, the catalyst could easily be applied to next generation energy devices," says lead researcher Dong-Gyu Lee.
The team are now looking to commercialise their development.
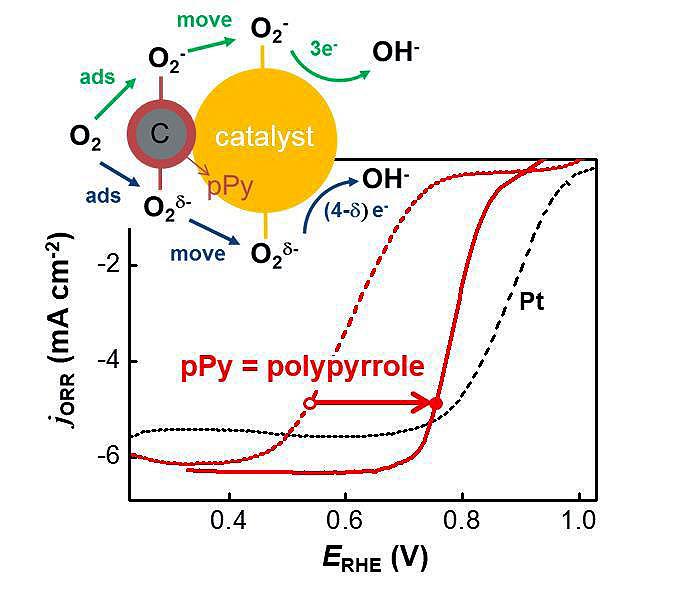
The oxygen reduction reaction at the anode of the cell is divided into four stages. The first step, which is the slowest, involves the addition of polypyrrole to improve the catalytic reaction.







